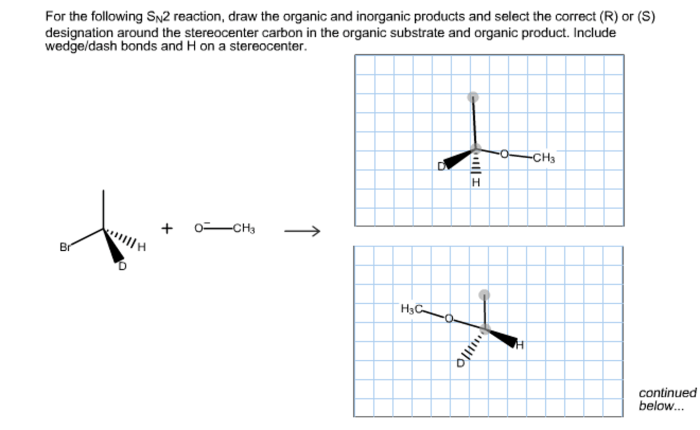
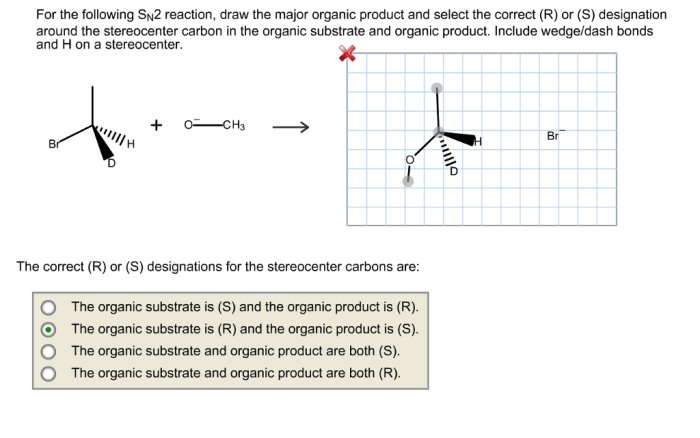
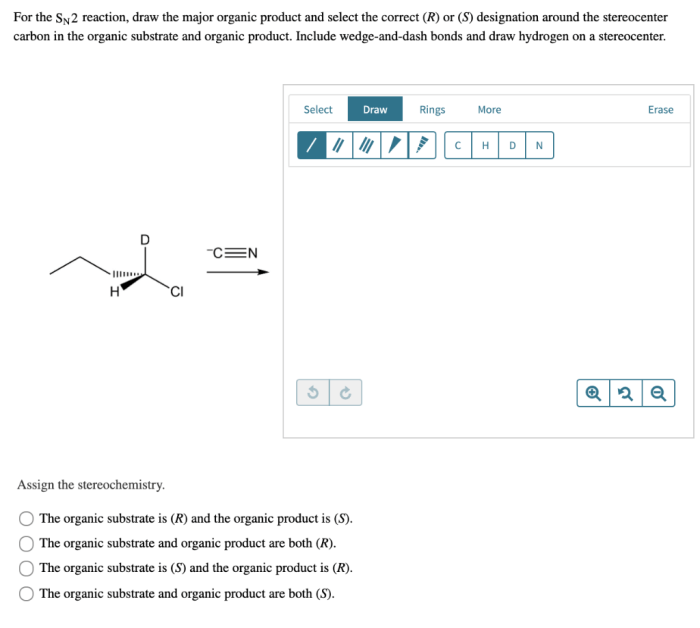
For the sn2 reaction draw the major organic product – In the realm of organic chemistry, the SN2 reaction stands as a cornerstone, renowned for its fundamental role in the formation of new carbon-carbon bonds. This guide delves into the intricacies of SN2 reactions, providing a comprehensive understanding of the factors that govern product formation and regioselectivity.
At the heart of SN2 reactions lies the concept of the major organic product, the predominant species formed during the reaction. Identifying this product requires a keen understanding of nucleophilicity, electrophilicity, and the steric and electronic effects that influence the reaction pathway.
SN2 Reaction Overview
The SN2 reaction is a substitution reaction in which a nucleophile (Nu) attacks an electrophile (R-X) and replaces the leaving group (X). The reaction proceeds through a single-step, concerted mechanism, with the nucleophile and leaving group departing and entering in a single concerted step.SN2
reactions are typically fast and occur with inversion of configuration at the electrophilic carbon. The rate of SN2 reactions is affected by several factors, including the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, and the solvent.
Major Organic Product Identification
The major organic product in an SN2 reaction is the product that is formed in the greatest amount. The nucleophilicity of the nucleophile and the electrophilicity of the electrophile play a major role in determining the major organic product.In general, the more nucleophilic the nucleophile, the faster the reaction will occur and the more likely the nucleophile will replace the leaving group.
Similarly, the more electrophilic the electrophile, the faster the reaction will occur and the more likely the nucleophile will replace the leaving group.
Regioselectivity in SN2 Reactions
Regioselectivity is the preference for one reaction pathway over another. In SN2 reactions, regioselectivity is determined by the steric hindrance around the electrophilic carbon.The nucleophile will attack the electrophilic carbon that is least hindered. This is because the nucleophile will have to overcome less steric hindrance to reach the electrophilic carbon.
Stereochemistry in SN2 Reactions
Stereochemistry is the study of the three-dimensional arrangement of atoms in a molecule. In SN2 reactions, the stereochemistry of the product is determined by the orientation of the nucleophile and the electrophile.The nucleophile will attack the electrophilic carbon from the side opposite the leaving group.
This is because the nucleophile will have to overcome less steric hindrance to reach the electrophilic carbon from this side.
Applications of SN2 Reactions, For the sn2 reaction draw the major organic product
SN2 reactions are used in a variety of organic synthesis reactions. One common use of SN2 reactions is to prepare ethers. Ethers are formed by the reaction of an alcohol with an alkyl halide.SN2 reactions can also be used to prepare esters.
Esters are formed by the reaction of a carboxylic acid with an alcohol.SN2 reactions are also used in a variety of other organic synthesis reactions, such as the preparation of alkenes, alkynes, and ketones.
FAQ Insights: For The Sn2 Reaction Draw The Major Organic Product
What is the key factor that determines the major organic product in an SN2 reaction?
The nucleophilicity of the nucleophile and the electrophilicity of the electrophile play a crucial role in determining the major organic product.
How does regioselectivity affect the outcome of an SN2 reaction?
Regioselectivity dictates the specific carbon atom at which the nucleophile attacks, leading to the formation of different isomers as major products.
Can SN2 reactions exhibit stereoselectivity?
Yes, SN2 reactions can exhibit stereoselectivity, resulting in the formation of specific stereoisomers as major products.